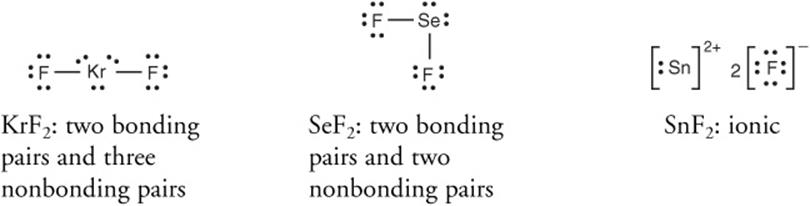
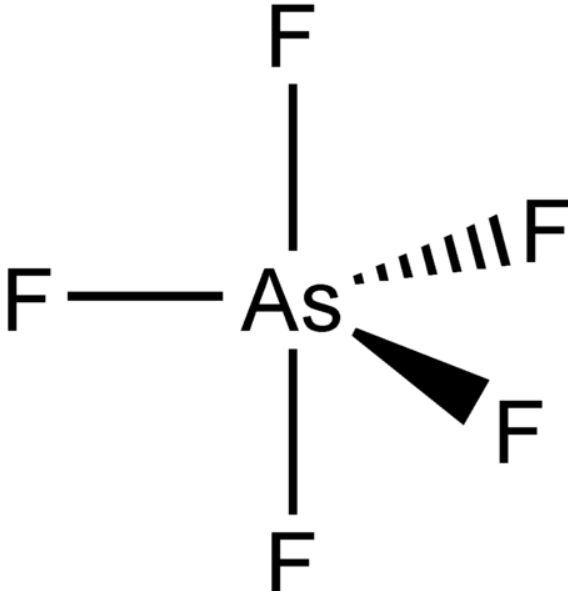
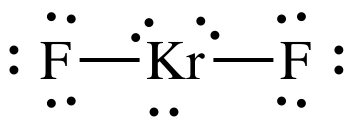✓ Solved: Consider the following compounds: CO2, SO2 KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, IF5, and...
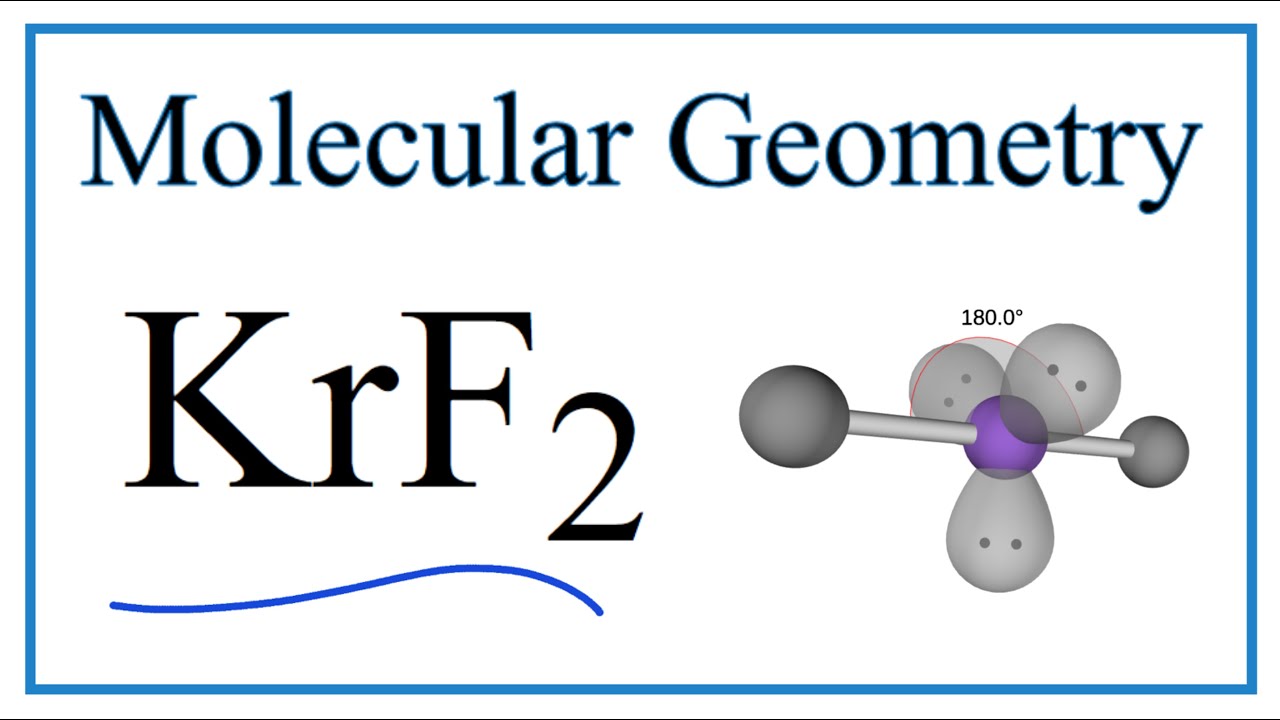
_krf2-molecular-geometry-bond-angles-and-polarity.jpg)
Lewis Structures, Introduction, Formal Charge, Molecular Geometry, Resonance, Polar or Nonpolar from pf3br2 lewis structure bond angles Watch Video - HiFiMov.co

SOLVED: Choose the solvent, water or carbon tetrachloride, which is going to be best dissolving agent each of the following? Explain why is it so? KrF2 CO2 SF2 MgF2 SO2 CH2O ch2-ch2
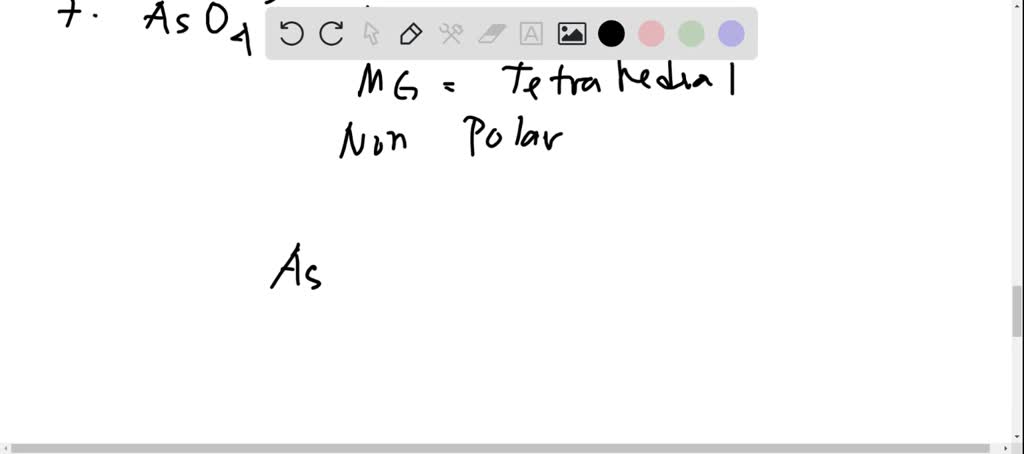
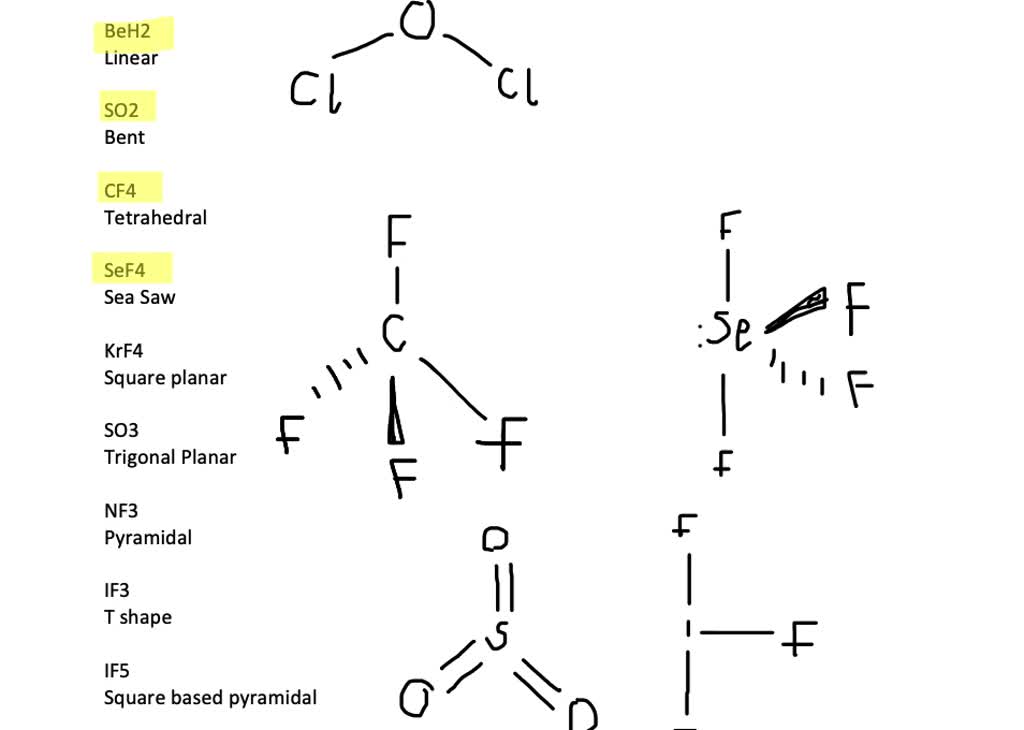
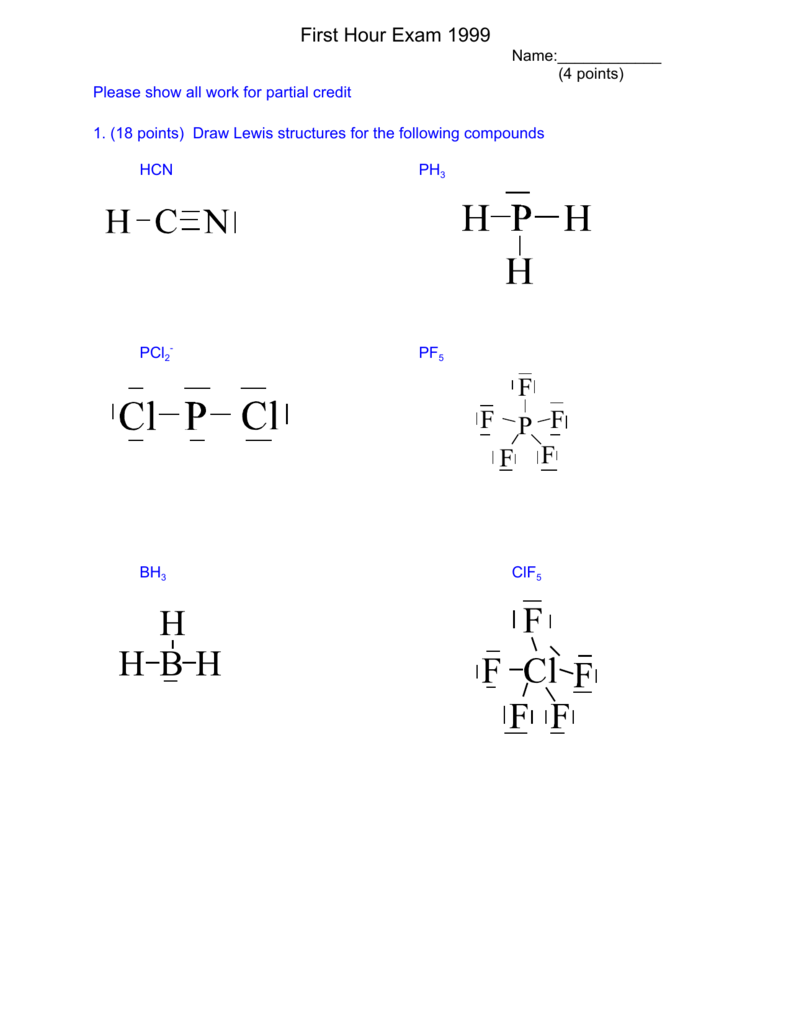
SOLVED: 3. Draw the Lewis structure for the following 10 compounds then label them with both electron domain geometry (EDG) and molecular geometry (MG) using your VSEPR reference sheet to help you.

Given Molecules SeO_2 KrF_2 ICI Lewis dot structure VSEPR pair polar or class Hybridization geometry geometry angle(s) nonpolar? | Homework.Study.com
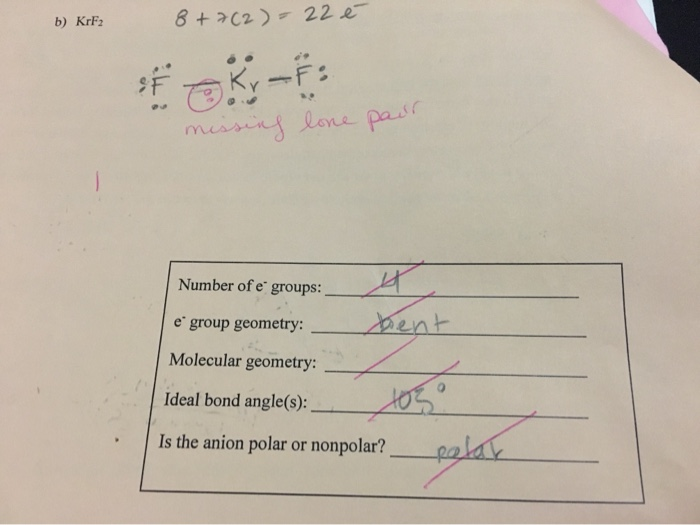
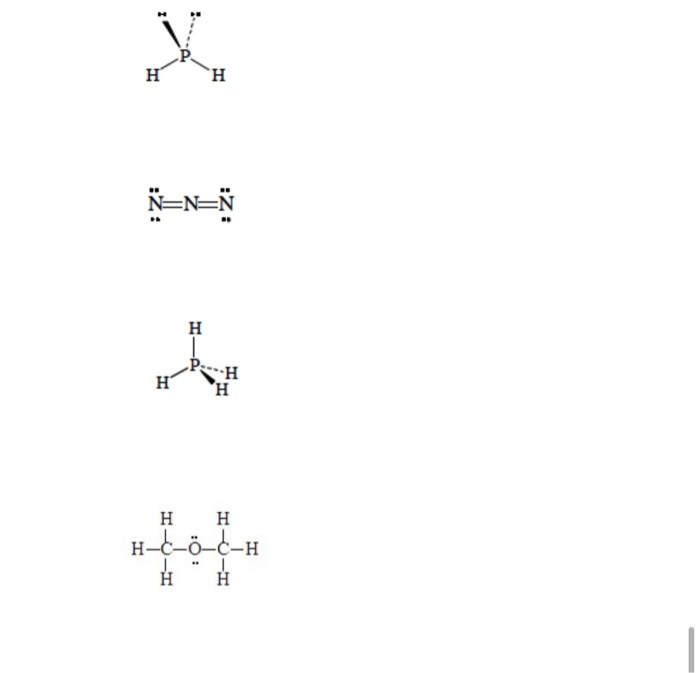



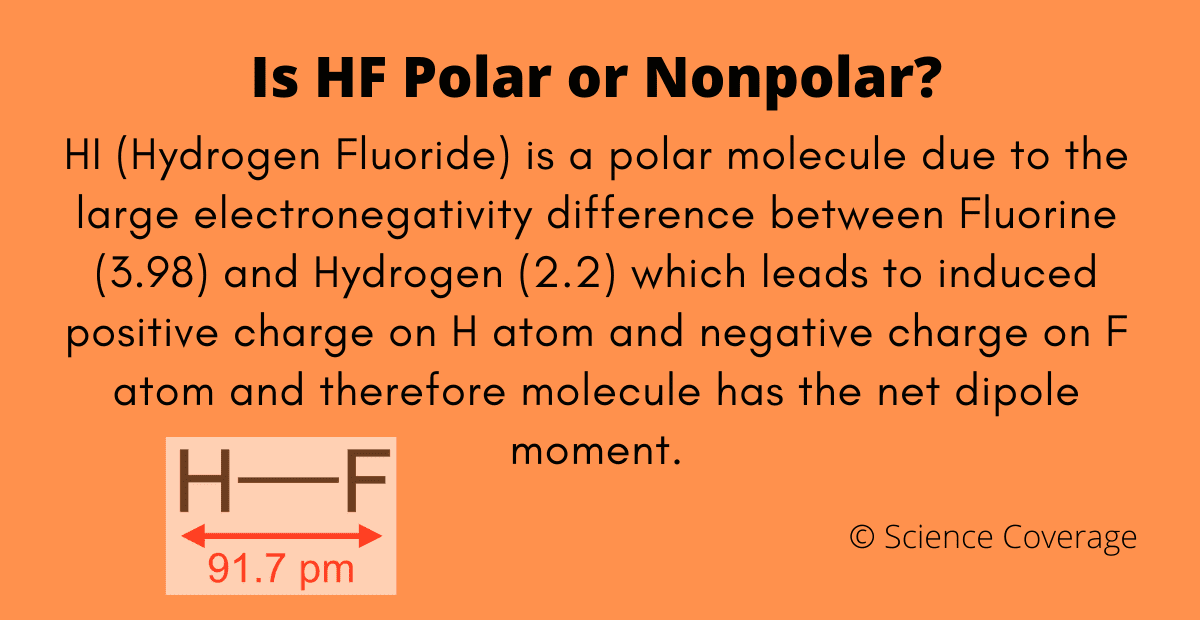
![Is NO2 Polar or Nonpolar? [Brief Explanation in simple terms] Is NO2 Polar or Nonpolar? [Brief Explanation in simple terms]](https://i.ytimg.com/vi/yZUkYlghpnk/maxresdefault.jpg)